Supramolecular Chemistry
Supramolecular Chemistry is a field that studies how molecules interact and form structures that are larger than individual molecules. Unlike traditional chemistry, which focuses on reactions between molecules, Supramolecular Chemistry looks at how molecules come together to create new, complex arrangements. This field explores how molecules can form various shapes and structures, such as capsules and networks, and how these structures influence their properties and functions. It helps us understand how to design and use these large molecular structures for various applications, from new materials to medical devices.
Back Home
When was it discovered?
Supramolecular Chemistry started to develop as a distinct field in the 1980s. It emerged from earlier work on molecular recognition and self-assembly. Key researchers, such as Jean-Marie Lehn, who shared the Nobel Prize in Chemistry in 1987, played significant roles in establishing and advancing the field. Since then, the study of how molecules can interact and form larger structures has grown, leading to new discoveries and applications in various scientific and industrial areas.
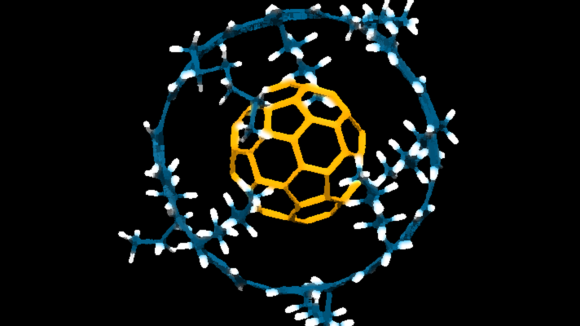
How does it help us?
Supramolecular Chemistry helps us by allowing us to design and create complex structures from smaller molecules. These large structures can have unique properties and functions that are not present in individual molecules. This knowledge is useful for developing new materials with specific properties, such as stronger and lighter materials or new drug delivery systems. It also helps in creating devices that mimic biological processes or improve industrial processes. Overall, it opens up many possibilities for innovation and problem-solving in science and technology.
How does understanding molecular interactions help us design new materials and devices?
Understanding molecular interactions helps us design new materials and devices by showing us how molecules can come together to form larger structures. These interactions allow us to create materials with specific properties, such as flexibility, strength, or conductivity. By knowing how to control these interactions, scientists can develop new technologies and improve existing ones. This can lead to advancements in many areas, including medicine, electronics, and environmental science, by creating more effective and innovative solutions.
Recap
What is Supramolecular Chemistry?
It studies how molecules form larger structures and interact.
When was it discovered?
Developed in the 1980s, with significant contributions from Jean-Marie Lehn.
How can it help us?
It helps create new materials and devices with unique properties.